Pay Attention, EU REACH regulation issued two amendments in succession!
Commission Regulation (EU) 2020/2081 of 14 December 2020 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards substances in tattoo inks or permanent make-up (Text with EEA relevance).
Commission Regulation (EU) 2020/2096 of 15 December 2020 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), as regards carcinogenic, mutagenic or reproductive toxicant (CMR) substances, devices covered by Regulation (EU) 2017/745 of the European Parliament and of the Council, persistent organic pollutants, certain liquid substances or mixtures, nonylphenol and testing methods for azocolourants (Text with EEA relevance)
一、(EU) 2020/2081 - New Entry 75 Clauses
According to EU research, inks or other mixtures used in tattoo products or permanent cosmetics usually consist of colorants and auxiliary ingredients, some of which have hazardous properties to human health. With the rapid growth of the number of tattoos in recent years, the toxic and harmful substances released from the skin metabolism are absorbed by the human body, thus causing great harm to the human body.

Therefore, the European Union has issued a new regulation (EU) 2020/2081, adding a new 75 clauses, which stipulates that tattoo products or permanent cosmetics shall not contain the following substances:
(a)Any substance classified in part 3 of Annex VI to regulation (EC) no 1272 / 2008:
1.Carcinogen class 1a, 1b or 2, or germ cell mutagen class 1a, 1b or 2, but excluding any such substances classified solely because of the effects of inhalation exposure;
2.Reproductive toxicant categories 1a, 1b or 2, but excluding any such substances classified as having effects solely due to inhalation;
3.Skin sensitizer category 1, 1A or 1b;
4.Skin corrosive category 1, 1a, 1b or 1C or skin irritation category 2;
5.Severe eye injury category 1 or irritating eye category 2;
(b)Substances listed in Annex II of regulation (EC) no 1223/2009;
(c)Substances listed in Annex IV of regulation (EC) no 1223/2009 with at least one condition specified in columns g, h and I of the table in that annex;
(d)Substances listed in Appendix 13 to this Annex;
(if one or more of the above substances are present, the mixture used for tattooing shall not be sold on the European market after January 4, 2022. Suppliers of tattoo products or permanent cosmetics need to pay attention!
In addition, the clauses also specifies the corresponding limits, labeling requirements, exempted substances and other information. Please refer to (EU) 2020/2081 for details or consult us for more information.)
https://eur-lex.europa.eu/legal-content/EN/TXT/?toc=OJ%3AL%3A2020%3A423%3ATOC&uri=uriserv%3AOJ.L_.2020.423.01.0006.01.ENG
二、(EU) 2020 / 2096 – Extensive revision of annex XVII to REACH Regulation
The European Union (EU) 2020/2096 amended items 3, 28, 29, 30, 43 and 46 in Annex XVII of reach regulation by deleting items 22, 67 and 68 on December 15, 2020. Details as follows:

1)Amendment to Clause 3
This entry contains several references to the use of the R65 label, which is one of the standard "r-phrases" which denotes special risks arising from hazards associated with the use of substances specified in Council Directive No. 67/548 /EEC. Since item 65 of the directive has been repealed. As the European Chemical Agency (ECHA) prepared a dossier under Article 69 of the regulation on 8 July 2015 and concluded that there was no need to propose amendments to the restrictions specified in this entry. As a result, paragraphs 6 and 7 had become redundant and were deleted.
2)Deleted clauses 22, 67 and 68
In REACH XVII, there are restrictions on pentachlorophenol and its salts and esters in item 22, decabromodiphenyl ether in item 67, PFOA and its salts and related substances in item 68. Due to the release of 2019/1021 (EU) in 2019, the EU pops (persistent organic pollutants) regulations impose more stringent restrictions on these substances. In order to avoid duplication, items 22, 67 and 68 in Annex 17 of the reach regulation were deleted from.
3)Deleted CAS and EC numbers of nonylphenol in item 46
The European Union has issued (EC) 552/2009, adding CAS number and EC number of nonylphenol in this clause. The purpose is to confirm the substance and help suppliers and law enforcement agencies to apply correctly. However, its intention is not to control only the CAS number and EC number of nonylphenol. Therefore, the CAS number and EC number of nonylphenol in item 46 are deleted to reflect the original intention of legislators to control all isomers of nonylphenol.
4)Revised requirements in items 28, 29 and 30
The Clauses 28, 29 and 30 of REACH XVII prohibit the placing on the market and use of substances classified as category 1A or 1b carcinogenic, mutagenic and reproductive toxicity (CMR). These substances refer to the European Union's 1272/2008 (EC) (referred to as CLP regulation), and this article shall also be updated due to the update of CMR 1A or 1b in CLP. However, the medical device regulation (EU) 2017 / 745 already contains provisions on CMR substances. In order to avoid double provisions, the equipment within the scope of 2017/745 regulation is exempted from the restrictions specified in article 28-30 of annex XVII of REACH regulation.
5)Updated the test methods for azo colorants and azo dyes in item 43
Test methods for azo dyes are listed in Appendix 10 of REACH XVII for use in item 43 of this Annex. The listed methods are obsolete and have been replaced by the latest test methods by the European Committee for standardization. Therefore, Appendix 10 is modified to apply to the test of azo dyes in Article 43.
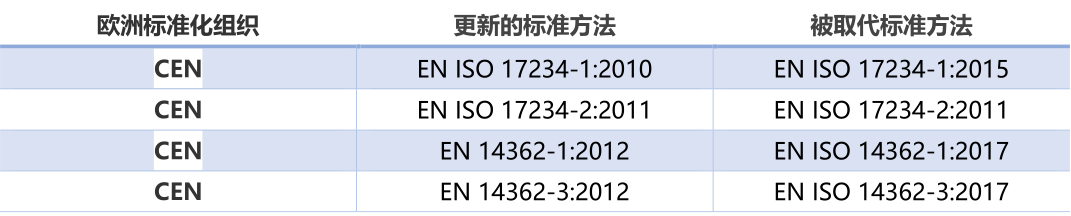
The above is the main content of the amendment (EU) 2020/2096, which will enter into force on the 20th day after its publication in the official journal of the European Union.
https://eur-lex.europa.eu/legal-content/EN/TXT/?toc=OJ%3AL%3A2020%3A425%3ATOC&uri=uriserv%3AOJ.L_.2020.425.01.0003.01.ENG
PDF Download:
